Types of ionising radiation
Alpha radiation
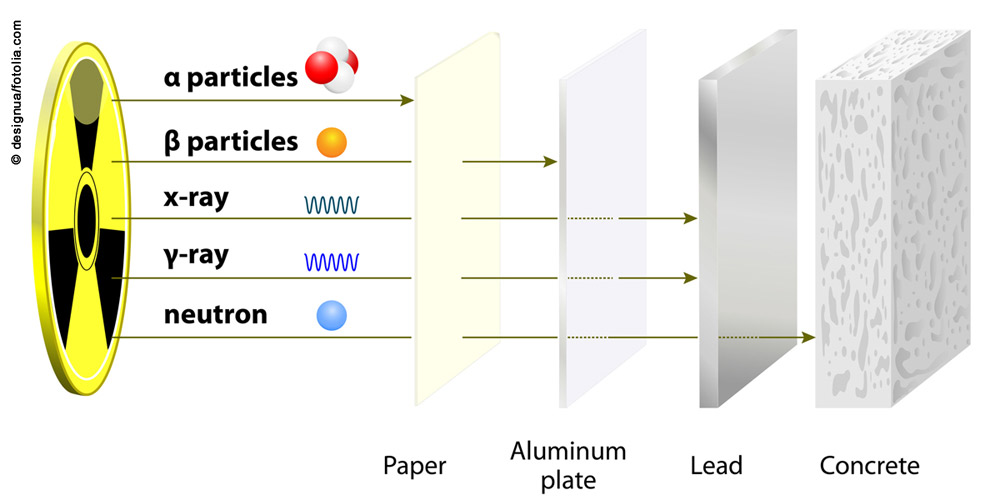
Alpha radiation is a form of particle radiation. Alpha particles are helium-4 nuclei: they consist of two protons and two neutrons. Alpha particles are absorbed very quickly by matter (by air and water, for example) and for this reason have only a very short range (a few centimetres in air; less than a millimetre in water). They can be shielded by a sheet of paper.
In the event of external exposure, alpha radiation can only penetrate the outer layers of human skin. Inhaling or ingesting alpha emitters – i.e. radioactive substances that emit alpha particles during decay – into the body (incorporation) can lead to a considerable radiation exposure. As alpha particles lose their energy within a very short distance, they cause particularly severe damage to tissue.
A typical and important example of the incorporation of alpha emitters is the intake of radon, a naturally occurring noble gas, and its progeny via inhaled air.
Beta radiation
Beta radiation is particle radiation that occurs when radioactive atomic nuclei emit (negatively charged) electrons or – less often – positrons (positively charged particles with the same mass as electrons) as they decay. Beta radiation is less easily absorbed by matter than alpha radiation and thus has a longer range: the penetrating power of beta particles ranges from a few centimetres to metres in air and a few millimetres to centimetres in soft tissue and plastic. Beta radiation can be shielded quite easily, for example, by an aluminium sheet a few millimetres thick.
Radioactive particles which emit beta radiation can also lead to a considerable radiation exposure when they are taken up by the body (incorporated) via inhaled air or food. In the case of external exposure, beta radiation can also damage tissue as it can penetrate the body, even if not very deeply. However, it loses significantly less energy over a certain distance than alpha radiation. Beta radiation is thus said to have a lower biological effectiveness than alpha radiation.
Gamma radiation
In the case of gamma radiation, energy is transferred as an electromagnetic wave. Electromagnetic radiation can be described in terms of its frequency or wavelength: the higher the frequency and the shorter the wavelength, the more energetic the radiation. Gamma radiation is at the high energy end of the electromagnetic spectrum, for example at the high frequency or short wavelength end.
Gamma radiation arises from the radioactive decay of atomic nuclei, often in addition to alpha or beta radiation. It penetrates matter very easily. Heavy materials such as lead and concrete are used for shielding.
Both external exposure and incorporation of gamma radiation are harmful to living organisms as it penetrates deep into tissue. However, its biological effectiveness is lower than that of alpha radiation, for example, as it transfers less energy to tissue over a certain distance.
X-radiation
X-radiation is also a form of electromagnetic radiation. Unlike gamma radiation, it is generated artificially when fast electrons are slowed down at the anode (positively charged electrode) of an X-ray tube.
The higher the applied tube voltage at which the electrons in the X-ray tube are accelerated, the shorter the wavelength and the higher the energy of the resulting X-radiation. When the X-ray machine is turned off, no X-radiation is generated.
Neutron radiation
Neutron radiation consists of uncharged particles (neutrons). Neutrons are mainly released during nuclear fission.
Neutron radiation is barely absorbed by air. Materials with the highest possible hydrogen content (e.g. paraffin, polyethylene, water) are used to initially slow down the neutrons. The decelerated (thermal) neutrons have to be captured by an absorber (such as boron or cadmium). Gamma radiation is emitted simultaneously and must be shielded by lead.
Neutron radiation interacts strongly with biological tissue (particularly the water molecules that it contains) and therefore has a high relative biological effectiveness.