Nuclear fission
The protons and neutrons are held together in the nucleus of an atom by the strong force there, which only operates over an extremely short distance. In order to induce nuclear fission artificially, energy is supplied to the nucleus from an external source. In commercial power reactors, neutrons are used to trigger nuclear fission.
Free neutrons can be captured by a nucleus. In most nuclear reactors in operation, the initial high energy of the free neutrons released during fission is reduced (moderated) until there is a high probability that they will be captured by a nucleus. If the neutron is captured, this produces a nuclide, whose nucleus is in an excited state; this means that it may no longer be stable. The nucleus of the atom may release excess energy. Depending on the nuclide, this may occur through fission.
Quelle: Stefan-Xp contribs / Vectorization: Wondigoma (contribs, Kernspaltung, adjusted value by BMUV, CC BY-SA 3.0)
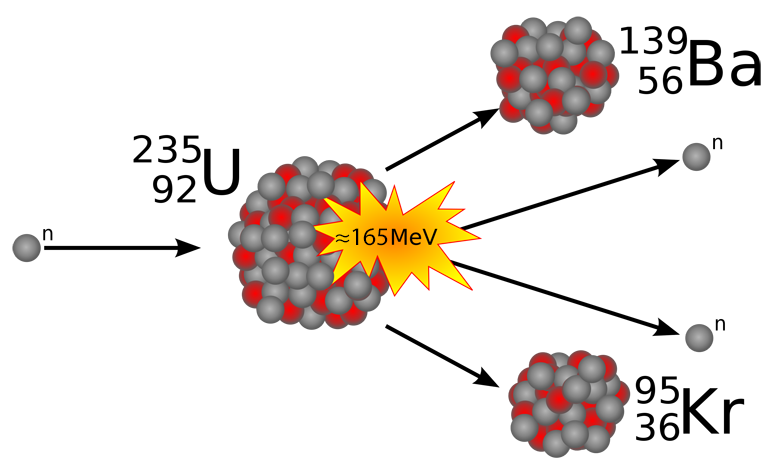
Uranium-235 is an example of a fissionable nuclide. If a thermal (decelerated) neutron is absorbed by a uranium-235 nucleus, it turns briefly into an excited uranium-236 nucleus. The uranium-236, in turn, splits into fast-moving lighter elements (fission products) and fast free neutrons are released (see previous figure).
The free neutrons can now induce fission in other nuclei, triggering a chain reaction. With no loss of free neutrons, the neutrons released by each fission event would increase and trigger more events, releasing even more neutrons and causing even more fission events. This reaction would then be continuously repeated at an uncontrolled rate. In nuclear reactors in operation, the number of free neutrons must therefore be regulated so that a controlled chain reaction can take place. Neutron poisons are used in order to lower this reactivity. Compounds of boron and cadmium – strong neutron absorbers – are often used.